What Changed and Why It Matters
The U.S. FDA is piloting generative AI to support its drug review workflow. The agency’s goal: faster document triage, smarter search across prior decisions, and tighter compliance tracking.
Why it matters: statutory review clocks remain the same, but the quality and speed of analysis can go up. That boosts the odds of “first‑cycle approval” for well‑prepared sponsors—a subtle shift with big downstream impact on funding, timelines, and market competition.
Here’s the part most people miss: AI won’t compress the law. It improves the odds you pass on the first try.
Zoom out and the pattern becomes clear. Regulators are operationalizing AI as a force multiplier for reviewers, while founders push AI deeper into discovery, clinical design, and dossier generation. Money follows the bottleneck. If the review gate gets less noisy, capital flows to teams who submit cleaner, more consistent data packages.
The Actual Move
Across reports, the FDA described an internal genAI assist to:
- Accelerate document review and evidence synthesis across massive submissions.
- Search prior reviews and guidance to improve consistency and reduce rework.
- Track and surface compliance issues earlier in the process.
Coverage also flagged open questions: transparency into the model and prompts, hallucination risk, auditability, data security, and how—exactly—the tool fits within scientific review and legal evidentiary standards.
Parallel signals in the ecosystem:
- Strategy lens: AI can’t legally shorten PDUFA or statutory timelines, but it can raise the probability of first‑cycle approval by catching gaps early and aligning to precedent.
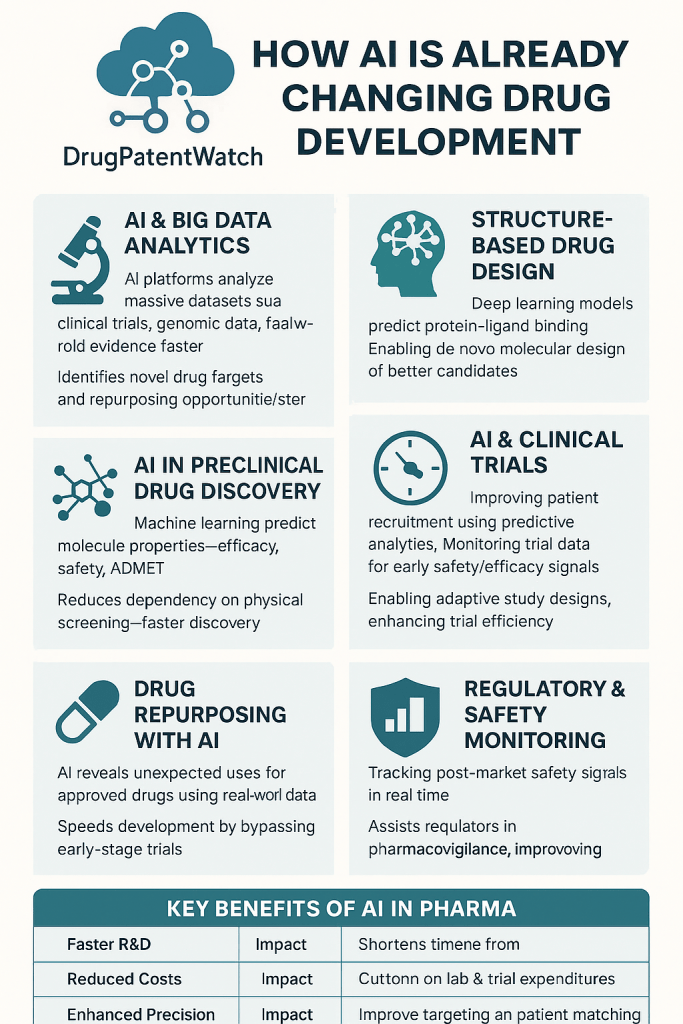
- Data opportunity: With access to FDA-scale data, AI could reveal patterns in trial design, endpoints, labeling, and safety that are hard to detect manually.
- Market reality: AI drug discovery is now a multi‑billion dollar segment, yet many companies face setbacks and funding pressure. Faster, cleaner regulatory paths could separate signal from noise.
Optimists expect review cycles to feel shorter in practice. Skeptics want proof the tooling is safe, explainable, and durable under real-world workloads.
The Why Behind the Move
FDA’s move fits a familiar pattern: use AI as decision support to cut search, summarization, and retrieval time—without replacing expert judgment.
• Model
GenAI excels at summarizing long documents, retrieving precedent, and harmonizing terminology. It struggles when outputs require perfect factual precision without human oversight. The FDA’s use case is squarely assistive.
• Traction
Regulators process hundreds of thousands of pages per application. Even small gains in reviewer throughput or error detection compound across CDER, CBER, and CDRH.
• Valuation / Funding
Investors reward anything that derisks the path to approval. If AI increases first‑cycle approvals, capital shifts from speculative discovery to “regulatory‑grade” tooling, clinical ops, and submission automation.
• Distribution
Winning startups won’t go direct to the FDA. They’ll integrate with CROs, eCTD vendors, safety systems, and regulatory consultancies. Distribution into existing GxP workflows beats net-new tooling.
• Partnerships & Ecosystem Fit
Expect alliances with EDC/CTMS platforms, real‑world data providers, and submission management tools. The moat isn’t the model; it’s access to structured, labeled, audit‑ready data and embedded pipelines.
• Timing
Internal AI at FDA creates a new equilibrium. Sponsors that align formats, ontologies, and evidence to how the agency searches and synthesizes information gain an edge.
• Competitive Dynamics
AI discovery remains crowded and cap‑intensive. A sharper regulatory layer is a faster path to revenue: protocol design, dossier generation, labeling intelligence, pharmacovigilance triage, and benefit–risk synthesis.
• Strategic Risks
Hallucinations, explainability, and audit trails are non‑negotiable. Tools must be Part 11/GxP aware, log prompts and outputs, and support reproducibility. Security, confidentiality, and bias controls are table stakes.
The moat is trust: verifiable pipelines, traceable provenance, and results that hold up under audit.
What Builders Should Notice
- First‑cycle approval is the KPI to build for, not “faster approvals.”
- The winning wedge is evidence quality: structured, consistent, precedent‑aware submissions.
- Distribution beats novelty. Embed in CROs and eCTD workflows to scale.
- Design for audits from day one: logging, lineage, validation, and change control.
- Align outputs to how reviewers search, not how scientists write.
Buildloop reflection
AI won’t change the rules—just who’s ready when the rules get applied.
Sources
- Forbes — FDA Embraces AI To Accelerate Drug Review Process
- Intuition Labs — Accelerating Drug Development with AI in the U.S. …
- Yahoo Finance — The AI drug breakthrough is taking a long time to arrive for …
- DIA Global Forum — Unearthing the FDA’s Treasure Trove: Using AI to …
- STAT — Five questions about FDA’s speedy rollout of AI for scientific …
- Pharmaceutical Executive — Can AI Accelerate Clinical Review at FDA?
- The AI Innovator — FDA Taps Generative AI to Speed Up Drug Review Process
- Reddit — F.D.A. to Use A.I. in Drug Approvals to ‘Radically Increase …